Areas of Expertise
In need of a solution? We bring years of experience to the table.
We accompany our customers in their daily work, but also in the special challenges posed by medical technology. We inspire our customers through our commitment, determination, authenticity, creativity, inventiveness, passion for plastics and flawless implementation.
Customised service
Our standard: individual care
As experts and system suppliers of medical devices and disposables, we attach the greatest value to precision, reproducibility and hygiene.
We strive to work out individually tailored solutions for each customer and their needs. We set the highest value on transparency, as well as adhering to jointly set goals and pre-determined schedules. In all of our actions, however, we never lose sight of the core of our work: improving patients' health and quality of life. Our above-average services help our customers and users to help others.

Project management
We meet our partners eye to eye level, implementing our customers' wishes and ideas with passion. Knowledge transfer, transparency and communication are particularly important to us in achieving the best result for each customer. In order to achieve optimal results for our customers in the shortest time possible, we localise all problems from the outset and define specific tasks in advance. To ensure maximum transparency, we integrate our digital document management into the complete process chain. Therefore, results and progress are available both immediately and over the long term.
When designing new products, our chief goal is to develop a complete understanding of the requirements and intended use. This is the only way to achieve a result that is appealing to users and patients alike, and which minimizes or even eliminates potential risks. In the next step, we perform an FMEA-supported individual functions and interfaces analysis. This helps us to identify and avoid potential sources of error at an early stage.
Injection moulds
When beginning a job, we first consider the potential risks to patients and users that might arise from the mold.
Only when we have analysed all aspects do we begin with the second step: designing and producing an injection mould fit for injection moulding production. Depending on the intended application of a component and the resulting requirements for hygiene, we offer a low-lubricant and - optionally - lubricant-free mould design. Our customers benefit from the Weber Group's decades of experience in plastics and injection moulding. When using lubricants or coatings, they are conceived with any special requirements in mind, and are of course approved for use in medical technology.

Clean room production
State of the art clean room production - it's what wezi-med stands for. ISO class 8, qualified specialist personnel and efficient working methods consistently guarantee top quality. In order to be able to offer our customers the highest level of product and service quality at all times and for any project, we manufacture according to GMP guidelines. In this way, trust and safety always go hand in hand. wezi-med's production area currently measures over 400 m², 125 m² of which is the clean room area. Welding, assembly and packaging processes are carried out there. Our plastic injection moulding machines have capacities ranging from 280 to 2,000 kN and are directly connected to the clean room via satellite placement. Injection moulded parts are handled and fed into the clean room completely automatically.
Clean room production facts at a glance:
- 125m² clean room working area
- ISO Class 8
- Injection moulding machines ranging from 280 to 2,000 kN, of which 1100 kN 2K
- Injection moulded parts are handled and fed into the clean room completely automatically.
- Welding, assembly and packaging of complex components
- Validated processes

Analysis
In the event of a customer inquiry, wezi-med first checks a number of parameters. The first step is a feasibility study. Here, we determine whether a product design that is suitable for plastics is possible in the first place. The next step is to check whether the product can be produced with an injection moulding machine. If these characteristics are positive, the material is selected and we determine whether or not any subsequent processes can be carried out in the clean room.
Plastic injection moulding
Plastics know-how since 1959 - this means the creation and design of better and more innovative products and solutions. We're never satisfied with the status quo, however. We continuously optimize and improve our working methods. This is the only way we can provide our customers not only with tailor-made solutions that match their ideas, but also with additional added value that they had not yet conceived of. We can do this for both one-component and two-component technology.

Packaging
- Sealing blister and Tyvek film with a sealing machine in the clean room
- Labeling according to customer requirements with CAB printer and Easylabel software
- Product traceability via barcode/QR code and UDI
- Blisterpack, Peel-off Tyvek film and labeling take place according to customer specification
- Validation and logging processes via the qualification phases DQ, IQ, OQ and PQ
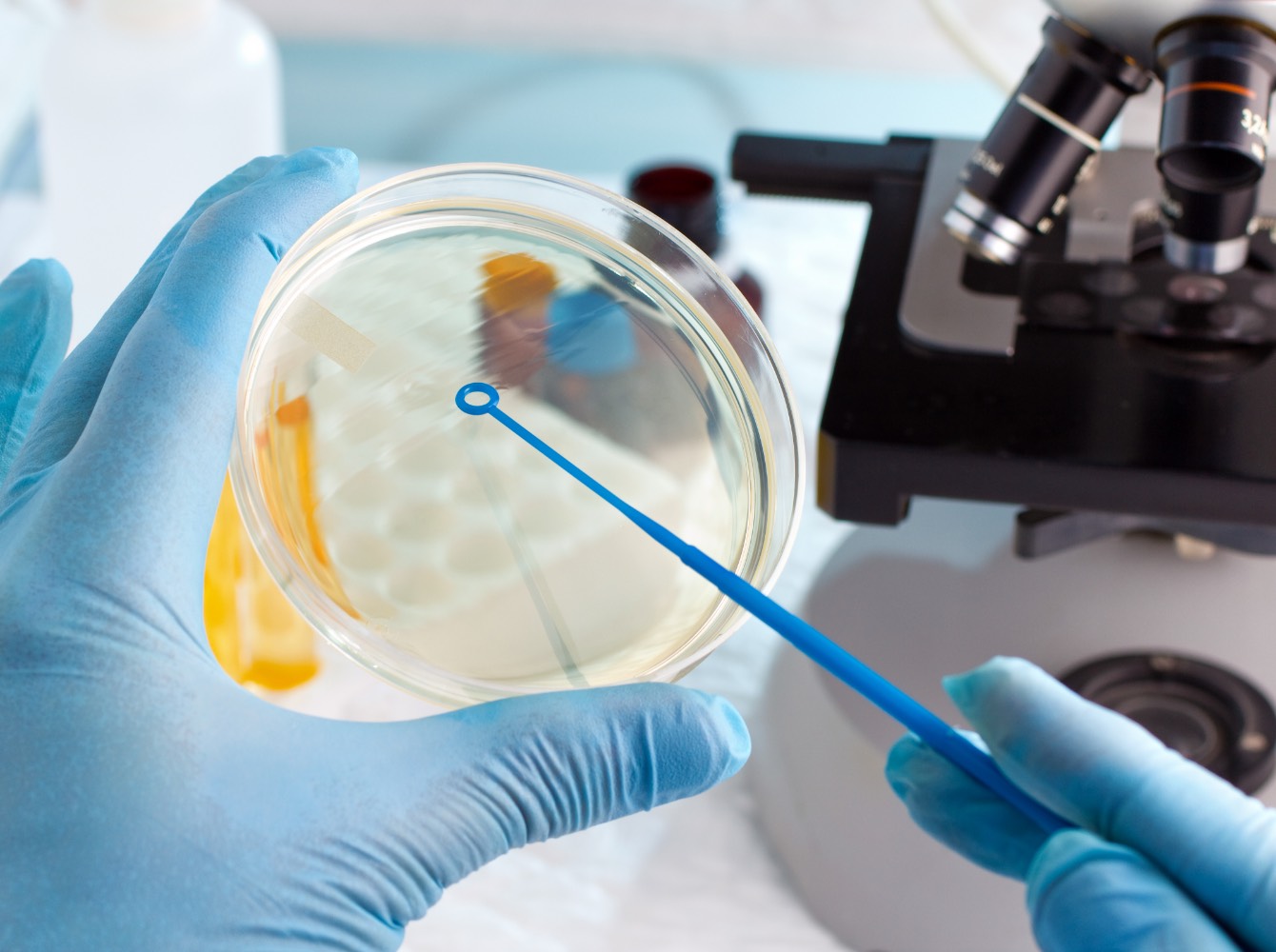
Sterilisation
Working alongside nationally renowned business partners, we offer various sterilisation procedures for medical devices. The best sterilisation procedure is determined on an individual basis for each application, in order to ensure maximum safety. Within the context of clean room production, we set the foundation for reliable sterilisation at an early stage, through microbiological controls and by applying an effective hygiene concept. Below is an overview of the sterilisation procedures we offer:
- Sterilisation with ethylene oxide
- Electron Beam Sterilisation (E-Beam)
- Beta- and Gamma-Ray Sterilisation
- Steam sterilisation











